A tale of shepherds and helices
A salt formed due to corrosion on a restored artwork features a structure that is known from the world of biology
The relief “Adoration of the Shepherds” by the Italian sculptor Giuseppe Torretti is disfigured by lumpy salt crystals. Now, a research group at the Max Planck Institute for Solid State Research in Stuttgart has established that the calcium acetate hemihydrate that makes up these efflorescences bears a similar structure to the protein collagen. The structural characteristics not only help prevent damage of this kind, but have also provided the researchers with interesting new ideas for bioinorganic chemistry.

The marble relief “Adoration of the Shepherds” is about 300 years old and has certainly had an eventful history. At the end of World War II, it was moved from Berlin to the Soviet Union, where attempts were made to repair the damage it had suffered during the war – including by using glue. In the early 1990s, the work by Giuseppe Torretti (1664–1743) was returned to the Museum for Byzantine Art in Berlin, where specialists removed the glue using ethyl acetate, an ester of acetic acid.
It is precisely this process that appears to have caused chemical changes in the surface, leading to the formation of white, needle-like efflorescences there a few years later. Experts from the Rathgen Research Laboratory in Berlin have identified them as chemical calcium acetate hemihydrate – that is, the calcium salt of acetic acid that also contains water. Over time, in other words, calcium from the marble had bonded with acetate ions from the solvent to form a new salt – and this substance has also been found on other old works of art.
However, it is only now that the compound’s crystal structure has been accurately determined. Restorers rely on structural characteristics as reference data so that they can identify corrosion products and explain the underlying process – as well as, ideally, preventing it. To determine the structure of the efflorescences in Torretti’s relief, Gerhard Eggert from the Institute of Conservation Sciences at the State Academy of Art and Design, Stuttgart, enlisted the help of Robert Dinnebier, head of the X-ray diffraction group at the Max Planck Institute for Solid State Research in Stuttgart. This is one of only a few groups in the world that specialize in complex structural elucidation even in samples that are only available in powder form and not as a single crystal.
Structural characteristics help prevent corrosion damage
The DFG-funded project “In search of structure” was also successful in determining this structure, revealing that the calcium ions arrange themselves along spiral-shaped chains – in other words, they form helices. The acetate ions, on the other hand, sit between the calcium ions and act as bridges. Altogether, three of these calcium strings wrap around one another in a similar way to the three strands of a plait.
Collagen also forms a triple helix

Helix structures of this kind are not uncommon in living nature. DNA, the molecule that carries genetic material, takes the form of a double helix – that is, a double strand wound into a spiral. According to Dinnebier, however, the structure that has now been identified bears a striking similarity to collagen proteins in particular, such as those found in the connective tissues of our body. “Collagen’s amino acid chains also form a triple helix,” the crystallographer explains.
However, the Max Planck researchers in Stuttgart could not use sample material from the efflorescences on Torretti’s shepherd relief for their structural analysis. “The corresponding X-ray diffraction technique requires quantities that you can’t get from an artwork of this kind,” explains Sebastian Bette from Dinnebier’s working group. The researchers therefore had to synthesize their own calcium acetate hemihydrate, which they did by leaving a calcium acetate solution in a covered terracotta pot at low atmospheric humidity for half a year. Over this time, the solution slowly passed through the pores of the pot wall and crystallized on the outside as calcium acetate hemihydrate. “We were then able to use this powder to carry out the X-ray diffraction analysis,” says Bette. This revealed the positions of the individual atoms relative to one another – and therefore the structure of the compound.
An unusually large unit cell
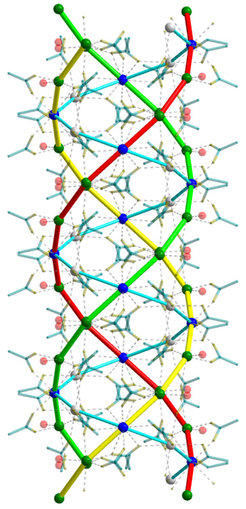
“It’s fascinating to discover a helical structural motif in a relatively simple compound such as calcium acetate hemihydrate,” says an enthusiastic Robert Dinnebier. In over 30 years of work on structural elucidation using X-ray diffraction, he has seen a lot of crystal structures and is no longer easily surprised. But he was amazed at the triple helix structure that has now been identified.
Apparently, the researchers were also surprised at the unusual size of the unit cell. Crystallographers use this term to refer to the smallest repeating unit of a crystal structure. With a volume of just under 12,000 cubic ångströms, a single unit cell in the analysed substance is several dozen times bigger than the most common unit cells of simple salts and contains 64 calcium ions. “That’s an incredibly large number for a relatively simple compound with only three components – namely calcium, acetate and water,” says Bette.
Possible template for proteins with helical structures
On the other hand, Robert Dinnebier now considers the structural similarity to collagen proteins entirely plausible: “Indeed, the most common amino acid in collagen is glycine.” As the simplest of all amino acids, this is closely related to acetic acid on the molecular level and therefore also to its salt, acetate. If you replace a hydrogen atom with an amino group in the acetic acid molecule, you get glycine. The researchers in Stuttgart now consider it likely that a helical calcium glycinate also exists. In principle, Dinnebier finds it “fascinating” that these “complex helical structures are quite clearly not limited to amino acid chains. Rather, they can also form elsewhere in certain circumstances.”
Due to its similarity to collagen, the researchers are now keenly interested in calcium acetate hemihydrate as a substance. Sebastian Bette says: “In evolution, collagen played a key role in the transition from single-celled to multicellular organisms and therefore also in the emergence of tissues.” Now, calcium acetate hemihydrate or a potential calcium glycinate could open up entirely new bioinorganic possibilities. “For example, we already know that the DNA double helix can be used as a template to cause other chemical compounds to crystallize in a helical structure,” says Bette. “It’s no great leap of the imagination to use the demonstrated triple helix as a template for proteins with helical structures, for example.”
KH


